The 3rd Cell Therapy Analytical Development Summit Europe– What was it about?
The 3rd Cell Therapy Analytical Development Summit Europe, was the premier event for technical professionals - analytical development, quality control, CMC, Tech Ops, and more!
Our 2024 attendees, learnt how to stay ahead by integrating cutting-edge technologies such as ML anomaly detection and automation in assay execution. Expert speakers also discussed the analytical life cycle and surmounting regulatory hurdles are equally critical, to showcase the unique qualities of your ground-breaking product.
Don't miss out on this opportunity in 2025 to hear from industry leaders, share your own insights and uncover ground breaking strategies to maximise product efficacy!
What You Missed in 2024:
Highlighting best practices for strategically placing control measures, emphasising continuous monitoring and adaptation to better address emerging risks with Tigen Pharma and Johnson & Johnson.
Ensuring product safety and quality through machine learning for enhanced quality control and anomaly detection with GSK and real time monitoring with Avobis Bio for revolutionised quality control.
Designing and craft cost effective assays for enhanced characterisation and streamlined regulatory compliance with Resolution Therapeutics.
Uncovering regulatory insights for navigating successful IND/IMPD applications and advancing analytical methods in both clinical and commercial stages for accelerated development with regulatory experts.
Discovering innovative approaches to classic methods including bypassing traditional methods for more accurate apoptosis assessments utilising real-time insights for point-of-care testing in cell therapy analysis and prioritising viability and functionality in storage methods with Tigen Pharma.
Who Could You Meet in 2025?
Connect with industry peers who share your passion for analytical development and are eager to explore the cutting-edge advancements. Get in touch here to discuss your future networking opportunities further.
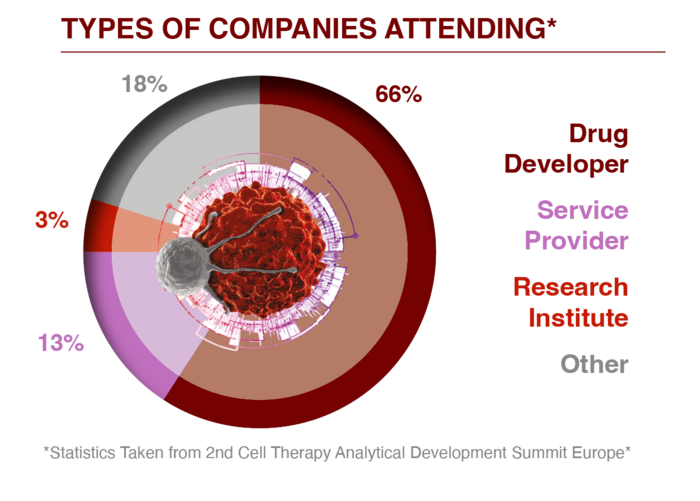
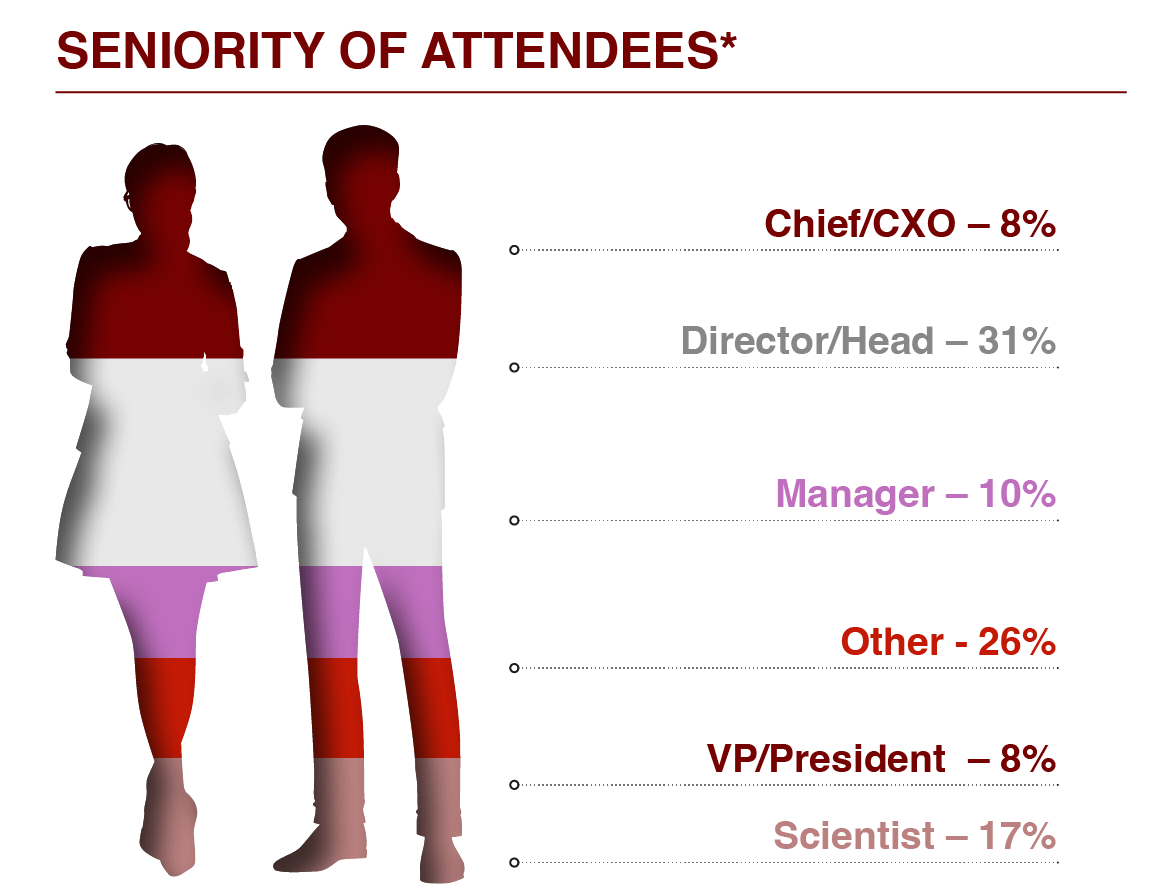
For further information or to access a past attendee list please enquire here.
What Your Peers Said:
"Cell therapy continues to change and expand the therapeutic field. To ensure the treatment is available, safe and potent, the analytical environment should coevolve."
– Amanda Versteilen, Associate Director, Quality Control Analytical, Bristol Myers Squibb (2024 Speaker)
“It is great to have a forum to discuss in depth, the technical and regulatory implications associated with analytical development and analytical change during development. I look forward to hearing about experiences as well as sharing my own. I learn something new every time I attend.”
– Sharon Lonhurst, Independent Expert (2024 Speaker)
