The 3rd Cell Therapy Analytical Development Summit Europe was a summit that brought together the brightest minds to revolutionise analytical development and quality control strategies for both autologous and allogeneic cell products. Like what you hear, seize this unparalleled opportunity to partner with us in 2025!
Why Partner?
This summit was the premier platform for showcasing your cutting-edge services to biopharma professionals eager for innovative solutions to enhance characterisation and improve product quality. By partnering with us in 2025, you will:

Position Yourself as an Industry Expert: Get in front of key decision makers and demonstrate your expertise through podium presentations, panel discussions or interactive roundtables.

Expand Brand Awareness: Broaden your company’s exposure with dedicated attendees from the likes of GSK, Bristol Myers Squibb, Johnson & Johnson, and many more.

Uncover Market Insights: Acquire invaluable insights into the solutions and services that biopharma companies will invest in to improve their analytical strategies in 2024. Armed with this market intelligence, align your offerings to meet the evolving needs of the industry, staying ahead of the curve.
Who Could You Meet in 2025?
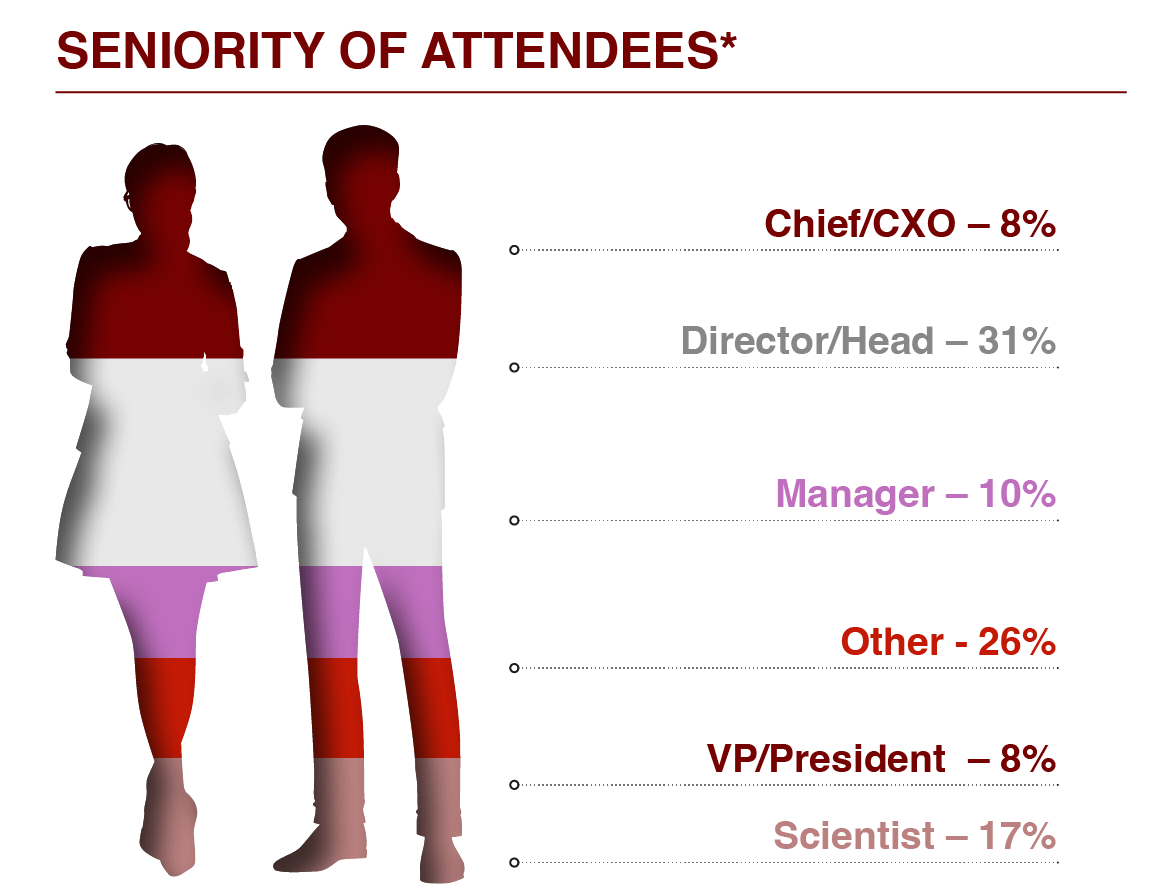
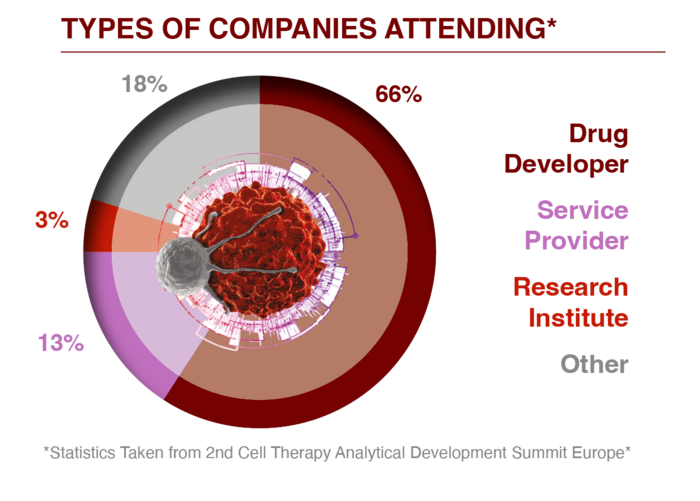
Capitalise on your chance in 2025 to connect with the technical leaders who rely on your equipment daily. Impress them with your expertise, gain their confidence, and get their buy in! You will meet VPs, Directors, Heads, and Scientists with specialisations in:
Analytical Development
Assay Development
Quality Control
Quality Engineer
Process Development
Bioinformatics
CMC
and more!
Want a Sample List of Past Attendees?
What Services Do Biopharma Need?
Service providers possess a wealth of knowledge and innovative equipment solutions that drug developers require. They are looking for support in areas including, but not limited to:
